Get Easy Health Digest™ in your inbox and don’t miss a thing when you subscribe today. Plus, get the free bonus report, Mother Nature’s Tips, Tricks and Remedies for Cholesterol, Blood Pressure & Blood Sugar as my way of saying welcome to the community!
4 Conditions that may impact your response to the COVID-19 vaccine
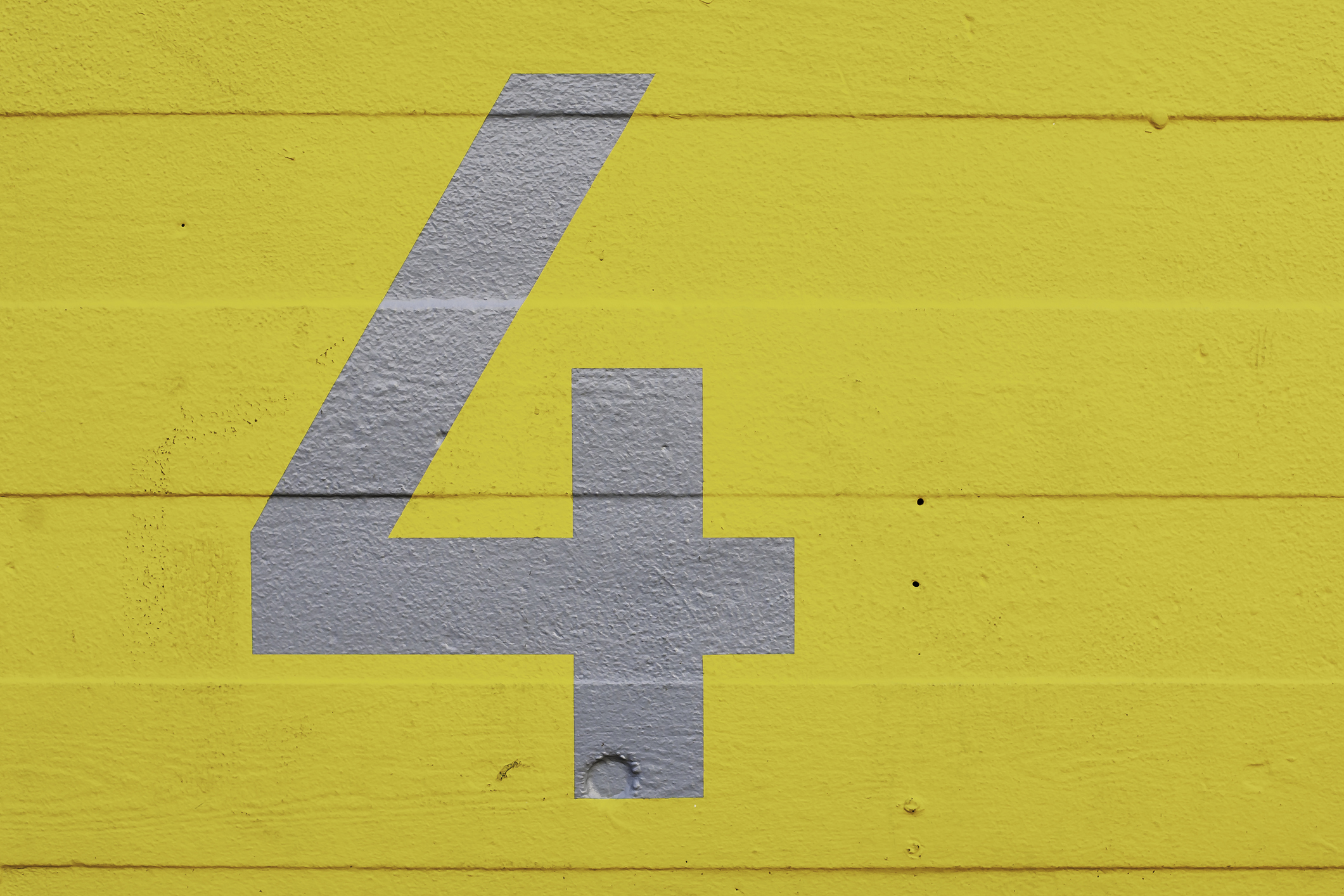
In the months since the COVID-19 pandemic hit, it’s become clear that certain underlying medical conditions can raise the risk of severe illness from SARS-CoV-2, the virus that causes COVID-19. Some of these conditions include chronic obstructive pulmonary disease (COPD), heart conditions like heart failure and cardiomyopathy, obesity, type 2 diabetes, cancer, chronic kidney disease, pregnancy, smoking and any condition that compromises the immune system.
What’s not as well-understood is how underlying medical conditions might affect a person’s response to the COVID-19 vaccines. Because the vaccines have only been in use for about a month, there isn’t enough data yet to clearly determine whether having an underlying condition could weaken or negate the vaccine response.
For now, health agencies are keeping a watchful eye and providing what information they can to help people figure out how well they might respond to the COVID-19 vaccine when their turn comes to take it.
Most conditions OK, but 4 Groups issued advisories
According to the U.S. Centers for Disease Control and Prevention (CDC), the mRNA COVID-19 vaccines from Pfizer- BioNTech and Moderna can be administered to people with underlying medical conditions as long as they have not had a severe allergic reaction to any of the ingredients in the vaccine. Right now, experts are recommending that people who have had an anaphylactic reaction to an injectable drug or vaccine containing polyethylene glycol or polysorbate speak with their allergists before getting either of the COVID-19 vaccines.
The CDC has issued specific advisories to people in the following groups so that they can make an informed decision as to whether to get one of the current COVID-19 vaccines….
People with weakened immune systems: While the CDC says people with HIV and those with weakened immune systems due to other illnesses or medication can receive an mRNA COVID-19 vaccine, they should be aware of the limited safety data available for their group. People living with HIV were included in the clinical trials, but safety data specific to this group is not yet available.
If people in this group do get one of the COVID-19 vaccines, one thing they should be aware of is the potential for reduced immune response to the vaccine. They will need to continue following all current CDC guidance to protect themselves against COVID-19.
People with autoimmune conditions: As is the case with the previous group, the CDC says people with autoimmune conditions can receive an mRNA COVID-19 vaccine. However, people in this group should be aware that no data are currently available on the safety of mRNA COVID-19 vaccines for them. While people with autoimmune conditions were eligible for enrollment in clinical trials, it’s unclear how many enrolled and what the safety data was for them.
People who previously had Guillain-Barre syndrome (GBS): The CDC says people who have previously had GBS can receive an mRNA COVID-19 vaccine. So far, no cases of GBS have been reported among participants in the mRNA COVID-19 vaccine clinical trials following vaccination. And with a few exceptions, the independent Advisory Committee on Immunization Practices (ACIP) does not include a history of GBS as a precaution to vaccination in its general best practice guidelines for immunization.
People who previously had Bell’s palsy: The CDC does note that cases of Bell’s palsy were reported in participants in the mRNA COVID-19 clinical trials. However, the Food and Drug Administration (FDA) determined these cases were not above the rate expected in the general population and have not concluded they were caused by vaccination. Therefore, the CDC says people who previously had Bell’s palsy can receive an mRNA COVID-19 vaccine.
Caution still warranted after vaccination
There have been questions about whether it is still possible to become infected and spread COVID-19 after getting the vaccine.
According to Jeffrey Bethony, a professor of microbiology, immunology, and tropical medicine at the George Washington University School of Medicine and Health Sciences who works on vaccines for parasitic diseases and HIV, “There is hope that they prevent transmission, but we simply don’t know enough about them yet.”
The CDC warns that even if people with underlying conditions choose to get vaccinated, they should continue to follow all current guidelines to protect themselves against COVID-19 until experts learn more about the protection the vaccines provide under real-life conditions.
That means continuing to follow the advice we’ve been hearing for months — including wearing a mask, staying at least 6 feet away from people not in your own household, avoiding crowds, washing hands with soap and water for 20 seconds or using hand sanitizer with at least 60 percent alcohol and following quarantine guidance after COVID-19 exposure.
Also, travel is not a good idea right now if you have an underlying medical condition. As far as travel goes, the CDC is advising everyone, regardless of medical status, to postpone any planned trips and stay home
Sources:
COVID-19: Vaccination Considerations for Persons with Underlying Medical Conditions — Centers for Disease Control and Prevention
COVID-19: People with Certain Medical Conditions — Centers for Disease Control and Prevention
COVID-19: Domestic Travel During the COVID-19 Pandemic — Centers for Disease Control and Prevention
Allergists offer reassurance regarding potential allergic reactions to COVID-19 vaccines — Massachusetts General Hospital
Can you spread COVID-19 after vaccination? Here’s what we know — Popular Science